Why Should a Top FDA Official Unilaterally Approve an Expensive Gene Therapy? The Case of Elevidys.
Peter Marks, the director of the Center for Biologics Evaluation and Research within the Food and Drug Administration (FDA), unilaterally approved Sarepta Therapeutics' gene therapy.
Elevidys, a gene therapy costing $3.2 million per patient, recently received traditional FDA approval for treating Duchenne Muscular Dystrophy. This approval followed a contentious regulatory process where Peter Marks overrode multiple review team decisions. Despite its high cost and potential toxicity, the clinical benefits of Elevidys remain uncertain. Here we examine the regulatory journey of Elevidys.

Gene Therapy for Duchenne Muscular Dystrophy (DMD)
Duchenne disease, or Duchenne muscular dystrophy (DMD), is a genetic disorder characterized by progressive muscle weakness and degeneration. It primarily affects boys, starting in early childhood, and is caused by mutations in the gene responsible for producing dystrophin, a protein essential for muscle function. Because it is a genetic disorder, gene therapy has always been an avenue of research. Over time, individuals with DMD lose their ability to walk and may experience heart and respiratory complications.
Delandistrogene moxeparvovec-rokl... Bless You! Let's call it Elevidys if we have no choice.
On June 20, the FDA “expanded the approval of Elevidys (delandistrogene moxeparvovec-rokl), a gene therapy for the treatment of Duchenne muscular dystrophy (DMD) for ambulatory and non-ambulatory individuals 4 years of age and older with DMD with a confirmed mutation in the DMD gene”.
According to the FDA: “While the large, randomized study of Elevidys failed to meet its statistical primary endpoint of improvement versus placebo in the NSAA, the FDA found the observations regarding the secondary endpoints and exploratory endpoints to be compelling and to indicate clinical benefit compared to placebo. These endpoints include improvements in time to rise from the floor, 10-meter walk/run, time to ascend four steps and creatine kinase levels.”
The Large Randomized Confirmatory Study Was… Negative!
The supplemental Biologics Application (sBLA) was supported by the double-blind study 301 trial.
“Study 301 was intended as a confirmatory trial “to verify and describe the clinical benefit, where there is uncertainty as to the relation of the surrogate endpoint to clinical benefit, or of the observed clinical benefit to ultimate benefit” as described in the approval letter dated June 22, 2023”
The trial was designed to show a benefit on a functional ability scale – the North Star Ambulatory Assessment (NSAA) total score – of 2.2 after 52 weeks. The study was negative, with an absolute difference in the least square mean change in NSAA total score between groups of 0.65 points (95% CI: -0.45, 1.74, p=0.2441).
The FDA Decision History
To recap:
The product was approved under the accelerated approval pathway in June 2023.
Accelerated approval was already made after Peter Marks decided against review team’s recommendation.
Post-marketing requirement included Study 301 which was negative
Sarepta submitted the data to convert the accelerated approval into a regular approval
The FDA commission decided against the conversion
Peter Marks – based on his own interpretation of key secondary endpoints – overrode their decision and the previous approval was converted into regular approval.
Below is an excerpt from the FDA Review team’s Clinical Evaluation:
Here is Peter Marks’s overriding decision:
Take Away Points
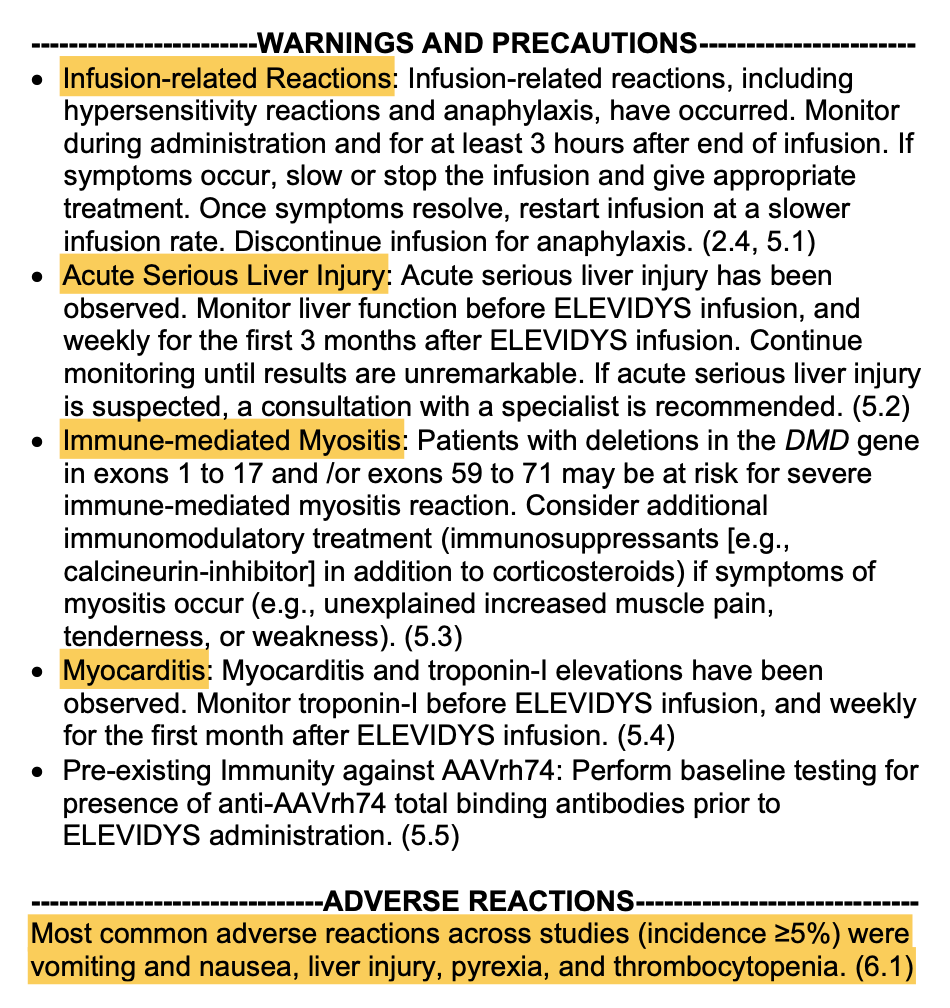
The product is one of the most costly drug throughout history, with $3.2 million dollar infusion, and is associated with potential severe side effets (see below, from the package insert)
The FDA approval history of this product is marked by disagreements between the review teams’ decisions and Peter Marks, the director of the Center for Biologics Evaluation and Research within the FDA. He overruled multiple review teams, resulting in Sarepta's product achieving traditional approval for at least one indication. Whether the product is clinically beneficial or not remains unclear.
We will closely monitor future regulatory decisions in that space. If you like this post, become of free or paid subscriber !








I'd be interested to know Peter Marks career history, and a detailed financial disclosure (that we'll never get) would also go a long way towards knowing his motivations to overrule his teams decisions and unilaterally grant approval for this product.
What I never understand is that ambulation as an outcome is the lesser of a DMD patients worries…what about the effect of dystrophin in cardiomyocytes…the heart failure is what is most concerning for DMD. For a gene therapy to be effective you have to fix all skeletal muscle (perhaps except extraocular muscle) and the heart. How is this feasible in postmitotic tissue.