What to expect from the LAURA trial presentation at ASCO-2024? (a PFS benefit? take it for granted)
Osimertinib "maintenance" after chemoradiotherapy in EGFR-mutated unresectable stage III lung cancer.
In this ASCO 2024 series, we provide background in expectation of the results to be presented during the upcoming ASCO 2024 meeting, which will take place between 31th May and 4th June. The annual congress of the American Society of Clinical Oncology is a key event in oncology. Each year, it attracts more than 30,000 attendees in Chicago.
EGFR-mutated lung cancer: short background.
EGFR is a proto-oncogene. When this gene is mutated, its oncogene state encodes an abnormally activated protein, leading to the uncontrolled replication of cancer cells. This phenomenon is characteristic of what are called “oncogene-driven” or “oncogene-addicted” cancers. EGFR-mutant lung cancers fall into this category. The frequency of such cancers varies, but they are more likely to affect patients who are from or live in Asia, are younger, non-smokers, and females more than males.
Small molecule oral tyrosine kinase inhibitors (TKIs) can block abnormal signaling, thus shrinking tumors and ideally improving clinical outcomes. After the discovery of first-generation EGFR-TKIs (erlotinib, gefitinib), the treatment landscape for patients with EGFR-mutated lung cancer has progressively been dominated by the third-generation EGFR inhibitor, osimertinib.
To recap:
in patients with advanced or metastatic cancers (mostly stage IV), it has demonstrated a better overall survival as the first-line treatment over gefitinib in the FLAURA trial. (HR = 0.80)
in the adjuvant setting (3 years!) of stage IB to IIIA cancers, it has shown a DFS and an OS benefit over placebo based on the ADAURA trial. (HR = 0.27 for DFS, HR = 0.49 for OS)
There is no doubt osimertinib is an advance. However, the ADAURA trial had extensive limitations including : inadequate initial staging, poor post-recurrence therapy, and more. Check out this analysis of the ADAURA trial by Vinay Prasad in the Drug Development Letter.
Here, the LAURA trial is really “filling the gap” between early stages (ADAURA) and the metastatic setting (FLAURA), focusing on stage III unresectable cancers, where patients had currently no indication to receive osimertinib.
Stage III unresectable lung cancers - current state
Those cancers are deemed unresectable, but are not considered metastatic either. It is also interesting to note that Progression-Free Survival (PFS) is the usual endpoint in this setting (PACIFIC and LAURA), when DFS or EFS are the endpoints in (neo)adjuvant trials. In other words, those cancers fall into an intermediate category between true adjuvant settings and clear metastatic situations.
STAGING: preferred recommended staging (according to NCCN guidelines) comprises a PET-CT and brain MRI
TREATMENT: for stage III unresectable lung cancer in general, the widely adopted approach is chemoradiotherapy, followed by 1 year of “adjuvant” durvalumab (an anti-PD-L1 immunotherapy). This is based on the PACIFIC trial, which demonstrated the addition of durvalumab improved survival in the overall population.
EGFR - SAME PATIENTS? NO. Those patients are very different from the overall population (younger, non-smokers, etc.).
EGFR - BENEFIT? in an unplanned, yet very informative post-hoc subgroup analysis from the PACIFIC trial led by Jarushka Nadoo showed NO BENEFIT from the addition of durvalumab in EGFR-mutated population (all caveats: small number of patients, post-hoc…).
EGFR - TOXICITY? severe immune-related adverse events are increased when osimertinib is given AFTER anti-PD(L)1 drugs.
As a result, there is a high expectation of the LAURA trial results, as the oncology community is not comfortable to simply apply the PACIFIC strategy to patients with EGFR-mutations, when data suggest no benefit, and with the known risk of severe toxicity when osimertinib is prescribed to patients upon progression.
A PFS benefit, take it for granted!
With this press-release from AstraZeneca, and the fact that the presentation is scheduled as a plenary, there is absolutely no doubt that the trial will show a PFS benefit. Because osimertinib is tested against placebo (and not an active drug), I also expect the Hazard Ratio to be low and significant, maybe around 0.3 or 0.4. Even if it’s 0.2, is that what I’m looking at ? Not really.
What to look at during LAURA presentation?
My main concern is that LAURA will broadly raise comparable concerns as ADAURA did, even if the setting is different. Is it better to treat all patients immediately after chemoradiotherapy, or to treat only patients at the time of progression?
These questions should be discussed with patients. The detailed breakdown of PFS events is also key in communicating the goal of therapy: are we avoiding local recurrences, distant recurrences, deaths, and in what proportion? Some patients may value their time WITHOUT therapy and, without a proven survival benefit, may prefer to choose surveillance, even knowing that the risk of progression is very high.
If a survival benefit is reported, did the control group had access to the best available care, including osimertinib? That’s the only way to derive a sound conclusion about survival.
Therefore, what will really pick my interest during LAURA presentation?
were the patients included in LAURA had proper staging? PET-CT? brain MRI? If not, and a fraction of included patients had metastatic disease, this would obviously favor the osimertinib group, and lead to worse outcomes in the control group.
what is the rate of patients not progressing in the control arm ?
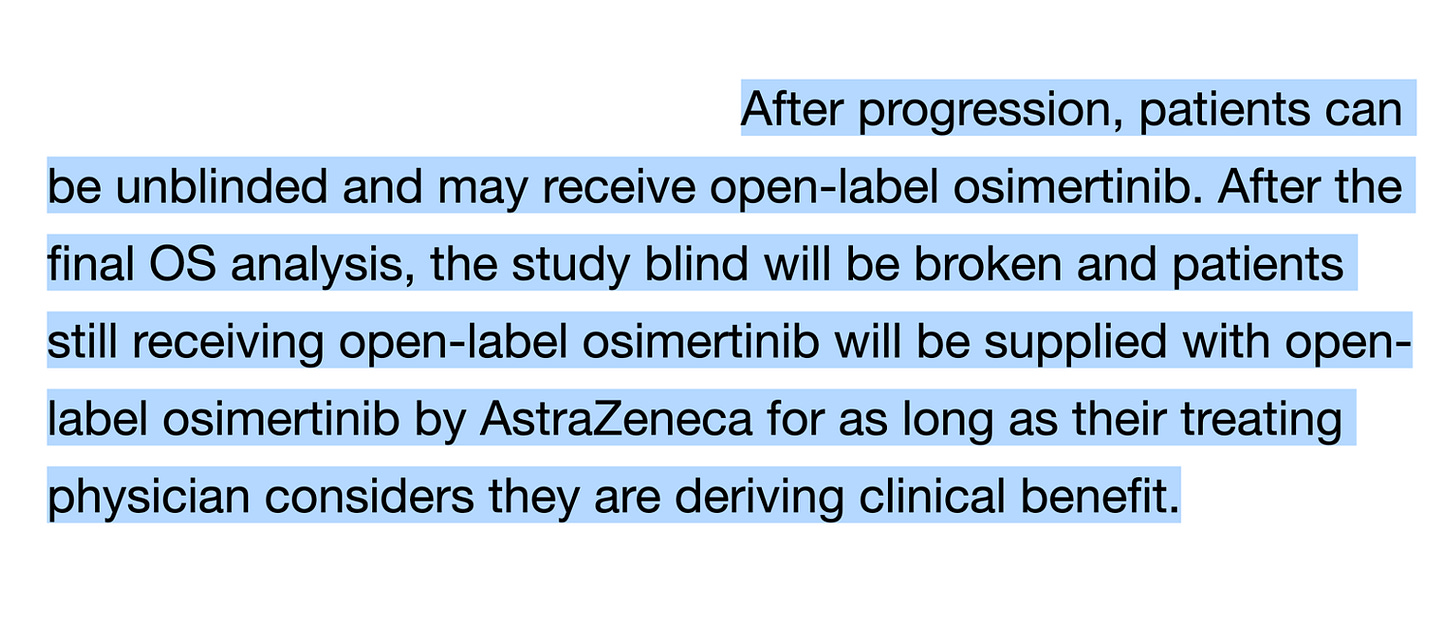
CROSS-OVER ! I would love to see survival data alongside with post-progression data. What was the access to optimal therapy in patients with progression? The study design ALLOWS a cross-over, but many countries lack access to the drug. The cross-over should not only be allowed, but should be mandatory and supplied by the company during the whole trial! And not only after the final OS (see below).
BREAKDOWN of EVENTS. I would love to see a detailed breakdown of the PFS events. PFS is a composite endpoint meaning an event can be local progression, distant progression, or death! Those events are grouped and the breakdown is rarely reported. Yet, it is vital to understand those, to better communicate the goals of therapy during real shared-decision making. This is even more relevant here, because osimertinib is highly effective upon progression.
what is the risk of developing post-radiotherapy pneumonitis in both groups? Was it increased in the osimertinib group?