THEOREMM - A Novel Framework for Cancer Registration Trials
"Trials in HEmatology and Oncology REviewed by Metaresearch Methods"
Today, the THEOREMM project and its protocol are launched with a publication in the European Journal of Clinical Investigation.
THEOREMM’s justification
Our proposal was developed to address the significant limitations that are currently missing in current trial assessments.
Several studies, including those of our research group, have shown key limitations in oncology and hematology trials, such as suboptimal control arm, poor postprogression or postrecurrence care. These limitations are critical because they cannot be corrected after the fact and can lead to biased trial results.
yet, low-value drugs come to market.
professional societies' scores, such as ESMO-MCBS or the ASCO Value Framework, do not incorporate the key limitations we describe.
GRADE is not well designed to assess individual registration trials.
If a randomized clinical trial yields a positive result with a new treatment, but this new treatment was tested against a suboptimal comparator, this element should be integrated and brought to the attention of regulators, prescribers, the public, and patients more systematically.
THEOREMM's idea is to integrate these often overlooked elements and make them accessible to a broad audience.
THEOREMM’s scope and methods
The scope is to assess registration trials, specifically those that lead to new drugs coming to market.
THEOREMM’s goals
increase awareness of key limitations potentially affecting clinical trials and to systematically integrate such limitations into a novel framework evaluating trials' results.
enable clinicians to make better-informed clinical decisions.
provide an educational platform for medical professionals, fostering an improved understanding of research methods.
be integrated in various aspects of current regulatory processes. Better-informed healthcare policies may justify resource allocation.
build trust and ensure relevance by patient and public involvement, as well as high-profile clinicians and trialists.
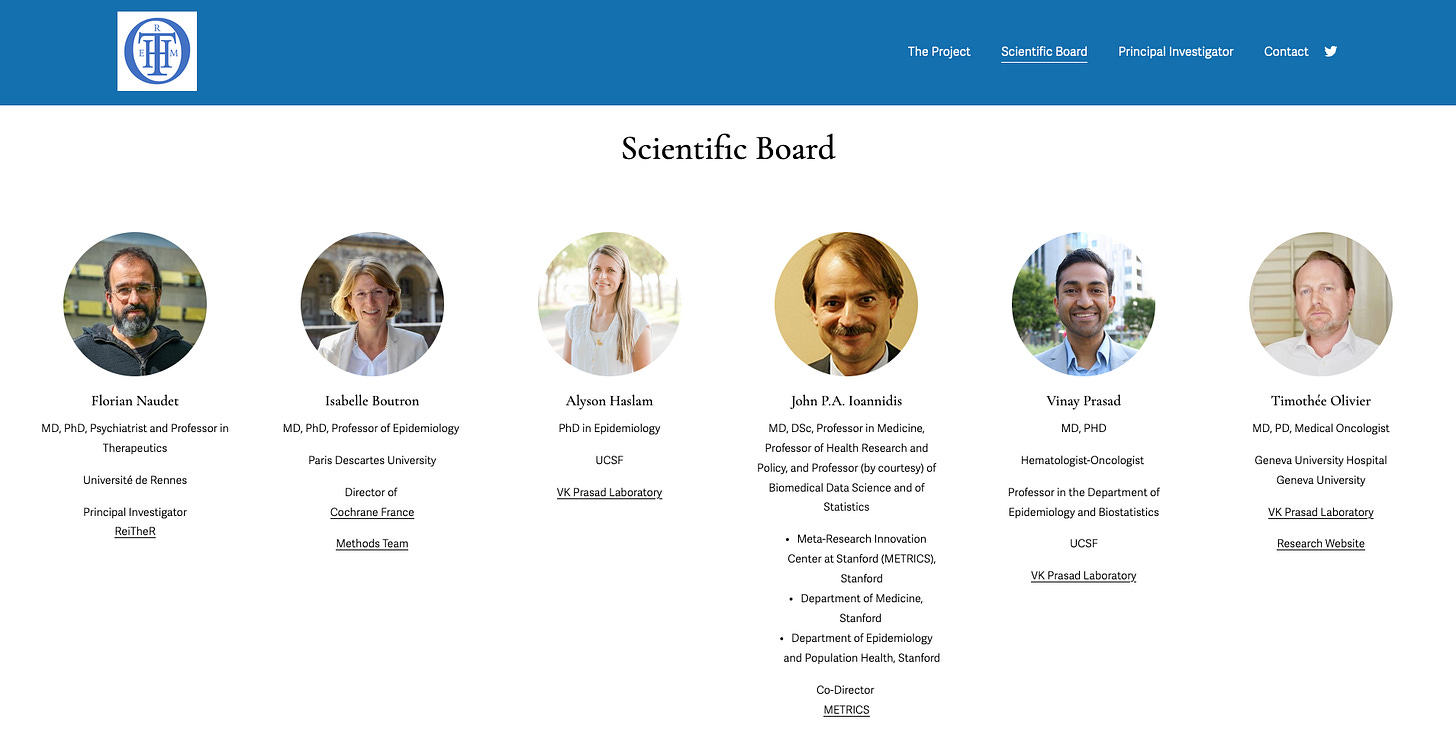
THEOREMM’s team !
Achieving such a goal requires the involvment of distinguished experts. Here is the scientific board of the project, composed of esteemed researchers who provided crucial insights for the upcoming stages. The project will involve trialists, the public, and patients extensively. Special thanks to Patricia Burke, a patient who contributed to the first publication, for sharing her invaluable perspective!
Check out the scientific board on THEOREMM’s website!
John P.A. Ioannidis: A pioneer in the field of metaresearch, he is the co-director of METRICS at Stanford.
Isabelle Boutron: Director of Cochrane France, a member of the SPIRIT-CONSORT steering committee, and leader of the METHODS group.
Florian Naudet: Principal Investigator of ReiTheR, conducting pivotal studies on reproducibility and research integrity.
Alyson Haslam, PhD: Key contributor to seminal projects supporting THEOREMM’s goals and philosophy.
Stay tuned with THEOREMM’s project updates!