Is speed the only thing that’s important when approving new cancer drugs? Some people would argue yes, slow approvals harm patients. You will remember that this was the case with antiretrovirals in the early 1990s and the reason the accelerated approval program began. But others would hesitate, and ask whether faster approvals equal better outcomes?
Obviously, it’s a balance, one that regulators understandably struggle with.
The FDA has been concentrating on speed, especially for cancer drugs. Founded in 2019, “Project Orbis” supports collaborative and simultaneous review of cancer drugs across eight countries to harmonize decisions. Given that drugs are almost always launched first in the US, the underpinning idea is that simultaneous regulatory review can speed up approval and access to promising cancer treatments in other countries. The partner countries include Canada, Australia, the UK, Singapore, Switzerland, Israel, and Brazil.
This year the Swiss regulator found that Project Orbis speeds time to regulatory approval. But we asked how clinically beneficial are these drugs? Also, health technology assessments are required in many partner countries given public systems to reimburse these drugs. Wait, patients and health systems need to buy these drugs….how much do they cost?
In our new work published in The Lancet Oncology, we asked whether the drugs reviewed through this program are better than the ones approved through other FDA pathways. We followed these drugs from FDA approval to health technology assessment bodies in the UK and Canada — the organisations responsible for reimbursement under public insurance plans, and estimated their list prices.
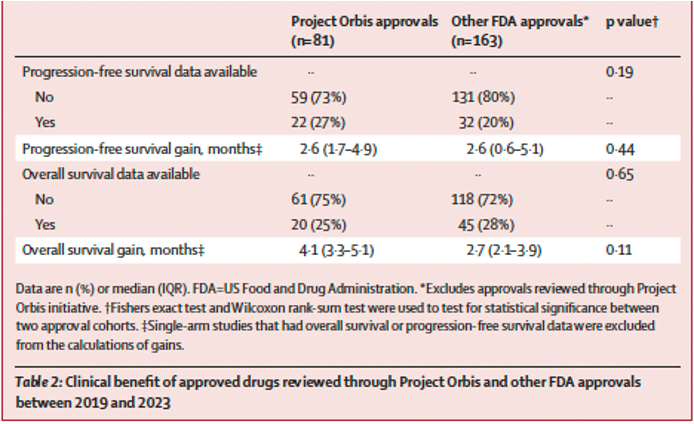
The clinical gains of drugs reviewed through Project Orbis are no different than those reviewed through other FDA programs.
The clinical benefits (defined as gains in overall or progression-free survival) of drugs reviewed through Project Orbis were not statistically different than among all FDA approvals over the same period.
It’s important to note that many of the drugs reviewed through Project Orbis are immunotherapies and may be high benefit for a subset of patients. But we do not know how these drugs were chosen. The FDA states that the criteria for drugs reviewed through Project Orbis are that cancer drugs must be clinically significant and ideally meet the criteria for the priority review pathway. However, there is limited transparency beyond this statement, raising questions about how the FDA selects drugs to be reviewed through this program and what clinical benefits patients receive. Governance will be key going forward as this program expands to other countries.
Second: while regulatory review times are decreasing, the time to health technology assessment decision is increasing
While the program has facilitated faster regulatory review times, health technology assessment decisions take a median of 350 days. Some drugs are ultimately not recommended for public reimbursement — only 33% and 72% were routinely available in England, Scotland, and Canada. This raises the question of whether Project Orbis speeds access in partner countries, as access is typically limited until after a favourable review.
The increasing time to decision is likely due to the high degree of uncertainty in the clinical evidence. About 40% of drugs were approved based Phase 1 or 2 trials. In the case of amivantamab for NSCLC (which was approved from a phase 1b trial), the health technology assessment bodies in England (NICE) and Canada (CADTH) said no. Same with tafastamab (DLBCL) and lurbinectedin (SCLC). One key question going forward will be how these organizations will fare under increased pressure from faster regulatory approvals.
Third: financial toxicity
We found that, on average, drugs review through this program were approximately $20,000 per month. While these estimates are list prices and will be lower, this finding highlights potential financial toxicity for patients and underscores potential burden on public health systems, such as those found in partner countries. Speeding up access to promising drugs is only advantageous when patients (and health systems) can afford them.
Project Orbis — the start of many other initiatives to globalize FDA standards?
Project Orbis is among an FDA-led push to converge regulatory standards across the world. Recently, Peter Marks announced that the FDA will build on Project Orbis for gene therapies. However, given our results, we ask: Will these initiatives be an opportunity for countries to harmonize up, or harmonize down?
Check out our full paper here