Postrecurrence treatment in neo/adjuvant trials.
Is it better to treat all patients early on, or to spare those who are already cured and only treat those who relapse?
Together with Alyson Haslam and Vinay Prasad, we have a new paper out in JAMA oncology ! We looked at all adjuvant & neoadjuvant drugs approved by the US Food and Drug Administration from 2018 to 2023 (May).
Neo/adjuvant setting
In cases of localized cancer treated with surgery, treatments administered before (neoadjuvant) or after (adjuvant) the operation aim to reduce the risk of recurrence, increase the chances of cure, and allow for less invasive surgery.
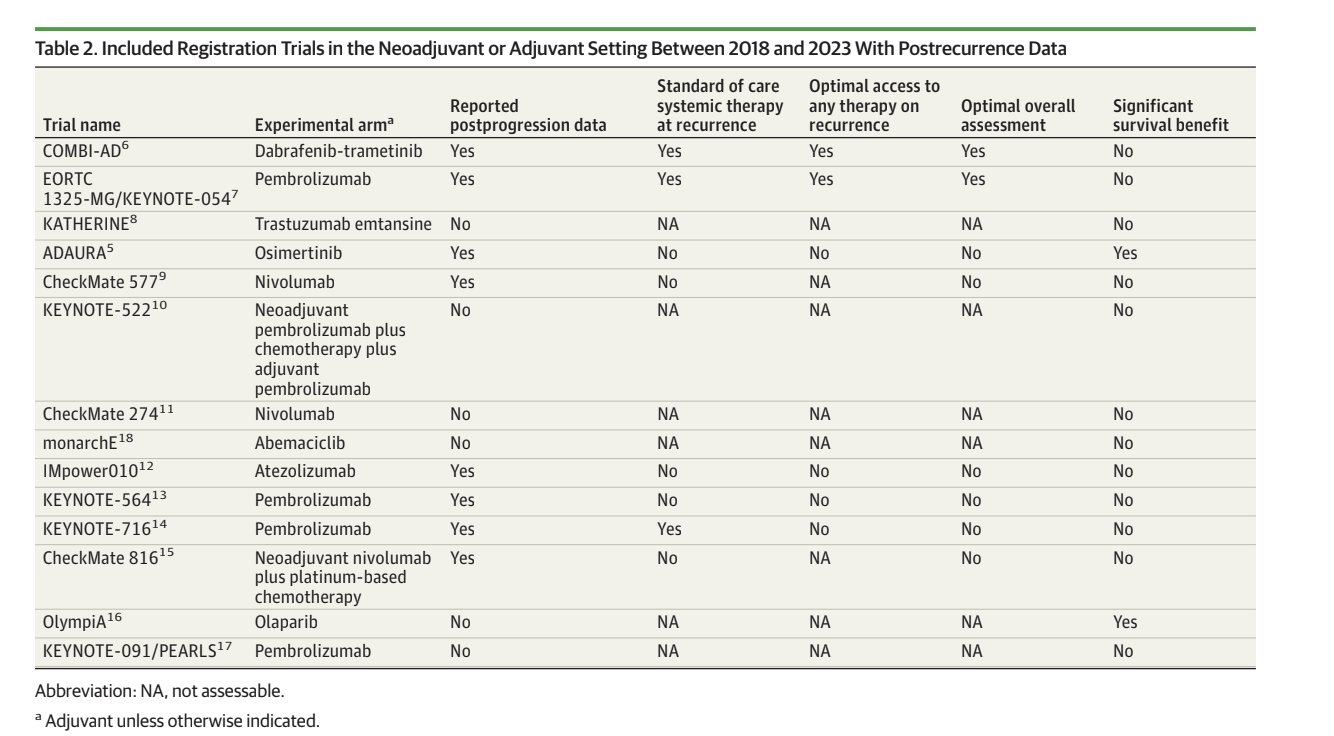
In recent years, treatments such as immunotherapy and targeted therapies are increasingly used in these situations. These are the drugs and trials we looked at. Very new, costly, potentially toxic agents. All improve DFS/EFS/RFS but only 2 improved overall survival at the time of our analysis (now it is 4).
However, these treatments have already proven their effectiveness in more advanced settings, when the disease recurs.
The question, therefore, is about the optimal strategy: should all individuals be treated at the early stage, with the certainty of treating some patients without benefits and exposing all to the risk of side effects, or should only those who will experience a recurrence be treated?
Results
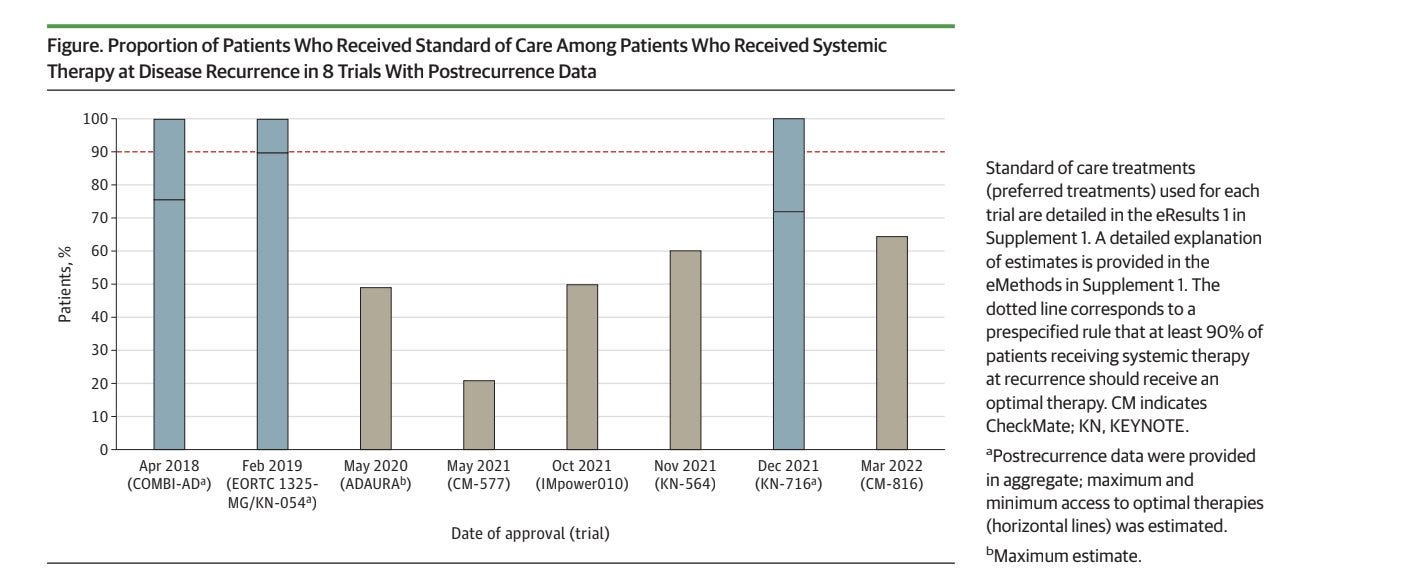
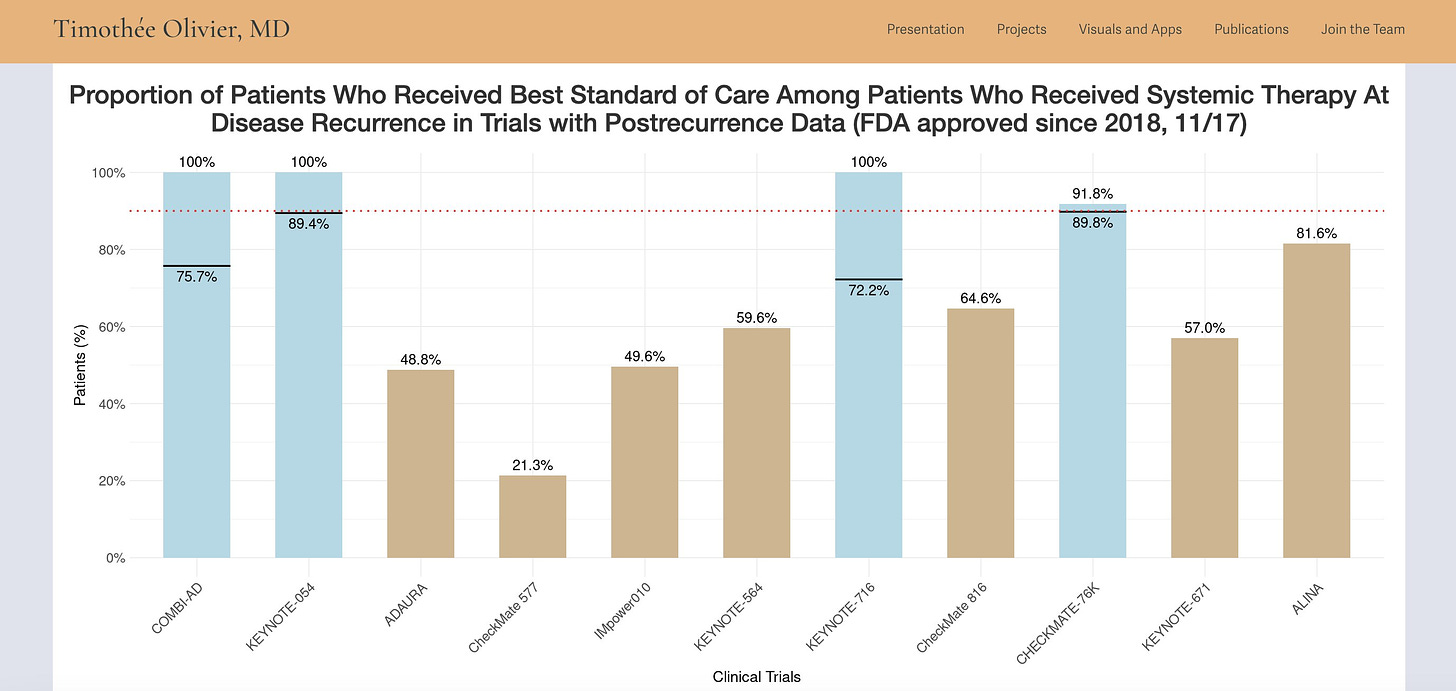
When reported, the rate of patients in the control arm receiving the best standard of care at relapse is very low. The blue bars indicate trials where reporting was insufficient, and we could only estimate an upper and lower bound of the percentage that received the standard of care.
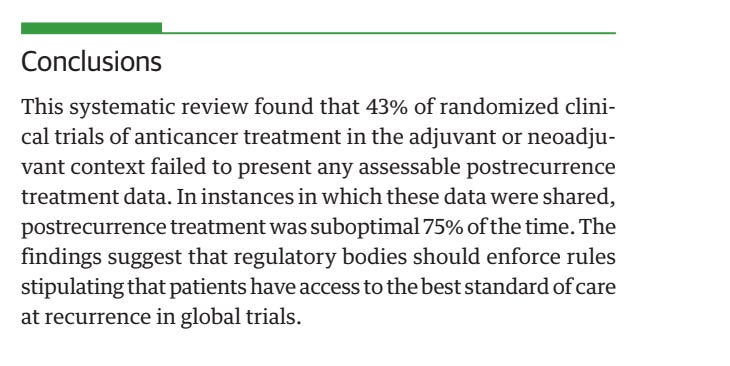
In 43% of cases, trials don't specify what control arm patients receive at relapse. When reported, it was suboptimal 75% of the time. In those cases, overall survival data is not reliable.
As more approvals come in that space, you can access updated figures and data on my research website here. Also, the full JAMA Oncology paper is available here.