Lu-PSMA and the need to 'educate' medical professionals about its use.
A dive into the VISION trial, PSMAfore trial, and the questionable practices of the sponsor.
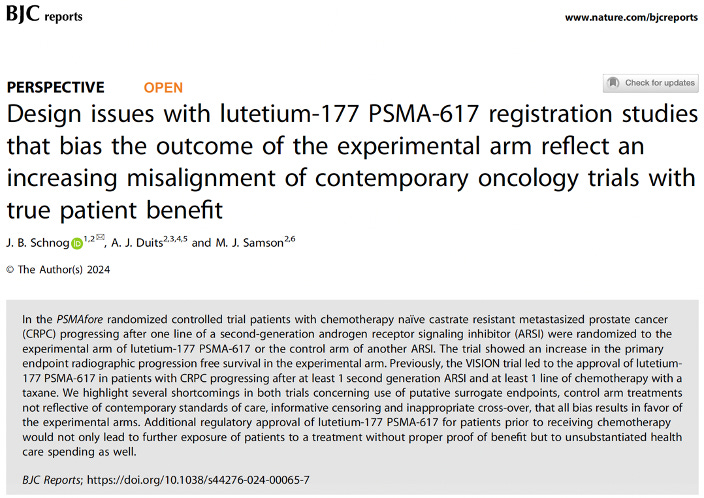
Background about “Lu-PSMA”
Lutetium-177 PSMA-617 is a radiopharmaceutical drug with activity (it shrinks prostate cancer) in patients with metastasized castration resistant prostate cancer (mCRPC) with positive prostate-specific membrane antigen (PSMA)-avid disease on positron emission tomography (i.e. PET-PSMA).
Based on a 4-month median overall survival (OS) benefit demonstrated in the VISION randomized controlled trial (RCT), Lutetium-177 PSMA-617 is FDA approved for treatment of patients with mCRPC progressing after at least one second-generation androgen-receptor signaling inhibitor (ARSI) and 1 line of taxane-based chemotherapy.
However, the demonstrated OS benefit in VISION trial has been heavily debated as the RCT suffers serious shortcomings that cast doubt as to the validity of the reported findings. Issues such as suboptimal control arm treatment (should have been cabazitaxel after progressing on docetaxel) with subsequent massive unbalanced early time point censoring in the control arm have been addressed online and in peer reviewed journals :
Timothée Olivier et al. free link here.
Our analysis after the PSMAfore trial.
Recently, results of the Novartis sponsored PSMAfore RCT have been presented, where mCRPC chemotherapy-naïve patients, after progressing on an ARSI, were randomized either Lutetium-177 PSMA-617 or another ARSI. An improvement in radiographic progression-free survival (the time until there is progression on an imaging study) was demonstrated. This RCT, just as the VISION RCT, suffers serious limitations as we have highlighted here.
In short:
lack of surrogacy: the surrogate endpoint does not predict a benefit in clinically meaningful endpoints such as OS.
suboptimal control arm: the control arm was unethical as treating patients with an ARSIs after progressing on an ARSI offers no benefit. These patients should have received docetaxel as control arm treatment (a proven life prolonging treatment for prostate cancer).
inappropriate cross-over: after progression on an ARSI in the control arm patients were allowed to cross over to Lutetium-177 PSMA-617. However, as per the VISION study, efficacy (living longer in a RCT) of Lutetium-177 PSMA-617 has not been demonstrated properly and patients are again prevented from receiving docetaxel. Therefore, should longer follow-up of the PSMAfore trial ultimately result in an OS benefit, this may very well be as the result of twice duping the control arm patients by not allowing the proper treatment with docetaxel.
In short, Lutetium-177 PSMA-617 is a drug with activity in prostate cancer but efficacy in mCRPC has not been properly demonstrated neither prior nor after docetaxel. It is an active drug but we have no idea if it improves OS compared to the current standard of care. Not speaking about the fact that Lu-PSMA is horrendously expensive.
Troubling statements from the company.
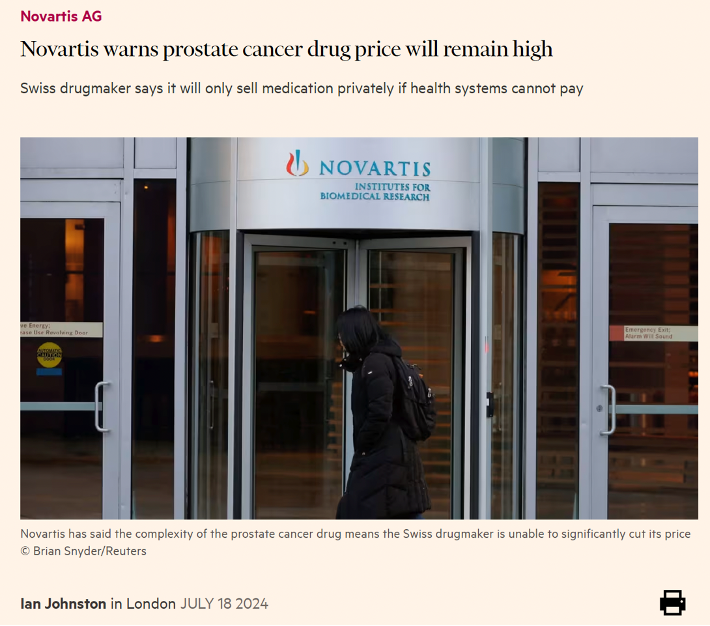
In a recent article published in The Financial Times, Novartis’s Chief Financial Officer argued that the complexity of the product justified not lowering the price of Lutetium-177 PSMA-617 (priced at $42,500 per dose, with patients often receiving 6 doses).
Here is a troubling statement found in the article. Sales in the second quarter of this year are lower than forecast. Therefore, "‘Novartis needed to “educate” medical professionals about the benefits of the treatment in order to increase referrals’, (i.e. ‘protect the value of the investment’).
In the VISION trial, when more than 50% of patients initially withdrew from the control arm due to ethical concerns, the trial sponsor initiated 'enhanced trial site education.' Despite these 'measures,' more than 1 in 6 patients still withdrew after being allocated to the control arm.
In other words, those who are reluctant to prescribe or approve a drug of which the value – i.e. the [clinically relevant] outcome of an intervention relative to its related costs – remains unknown will be ‘targeted for education’ with the aim of increasing Lutetium-177 PSMA-617 sales.
In the current system which combines a low threshold for approvals, questionable trial designs, and ubiquitous financial conflicts of interests, the statement made above underlines the ever-increasing importance of focusing medical education on proper critical appraisal of evidence.
A deep focus on teaching how to read scientific articles and interpret evidence within medical education would provide physicians with the most important tools to resist the proposed ‘brain washing’… oups sorry: ‘enhanced trial site education’ from the very organizations that sponsor poorly designed trials while simultaneously promoting their expensive products.












I attempted to share this article on FB
(fascist book)-they removed it....goes against community standards..... Censorship-enough said.
Novartis’ statement is more than troubling, it’s pablum. They have no hard evidence their overpriced drug helps patients live longer or better. They have evidence that it satisfies a surrogate endpoint. Surrogate endpoints are, by design, artificial goals. They may be biologically informative but they do not answer the question, “Does this drug help cancer patients live longer or better?” Clinicians should boycott this drug until it proves to be at least non-inferior to taxanes.