Low-dose dexamethasone in patients with newly diagnosed multiple myeloma.
The NCI - ECOG E4A03 trial showed longer survival and less toxicity with low-dose dexamethasone in newly patients with multiple myeloma.
FRIDAY’S GEM, EDITORS’ PICK
In this section, we present a paper that cought our attention, it can be recent, seminal, historical, a point of view, a commentary, a clinical trial, any publication that we feel is important!
We recently started this section with a work by our colleagues Ghulam Mohyuddin and Aaron Goodman about overtreatment in Multiple Myeloma. The authors advocated for de-escalation trials. In today’s post, we choose a notable example of such a trial, which led to the publication of the ECOG E4A03 trial in 2010, led by Vincent Rajkumar. This remains a seminal and practice-changing trial in the field, and an example of what we should be aiming for in modern oncology.
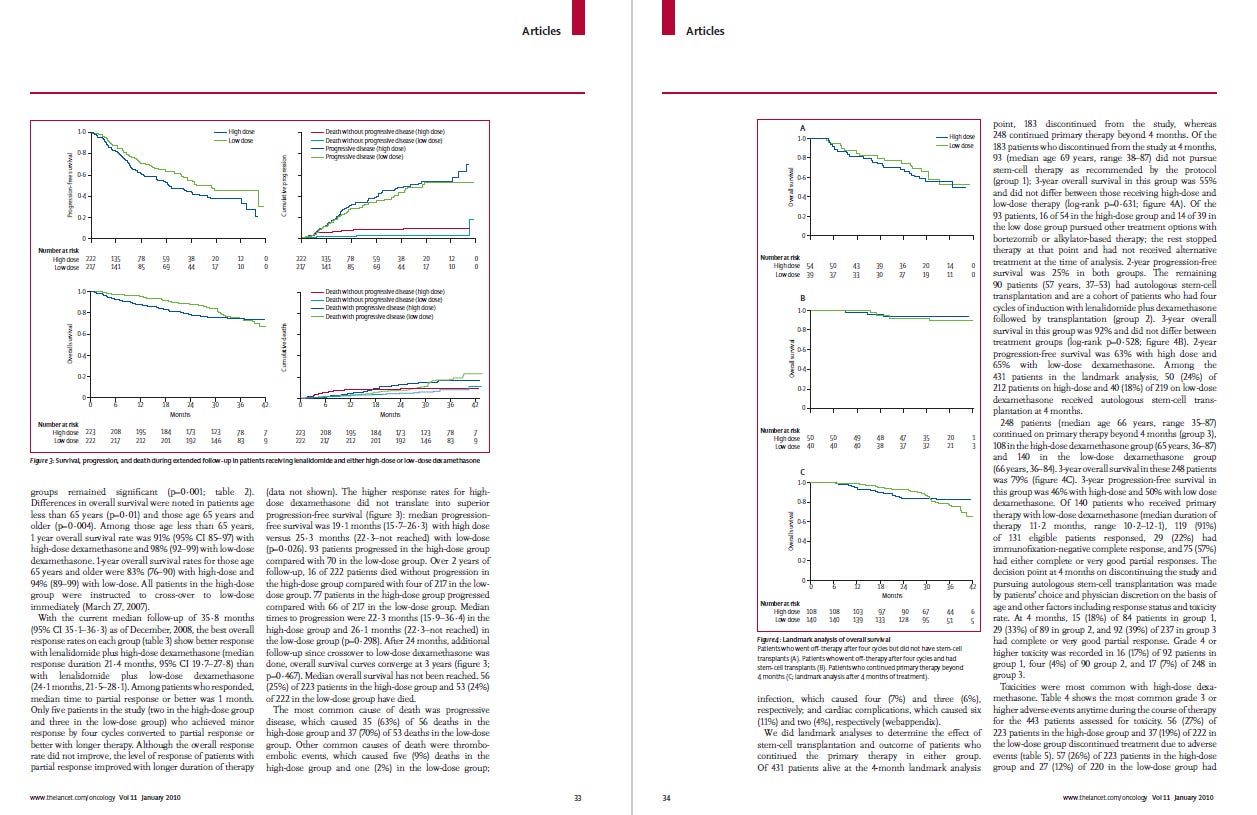
The NCI - ECOG E4A03 trial: what is the best dexamethasone dosing? A day-to-day question.
This was a non-inferiority open-label study where 445 patients with symptomatic untreated multiple myeloma were enrolled. All patients received lenalidomide. Patients randomized to the high-dose steroids group received dexamethasone 40 mg from day 1 to 4, 9 to 12, and 17 to 20 of a 28-day cycle (total of 12 doses per cycle). Those in the low-dose group received dexamethasone 40 mg on days 1, 8, 15 and 22 (total of 4 doses per cycle)
Main results, the low-dose group showed :
better short-term overall survival
less toxicity
The trial still stands as an excellent example of an academic study addressing a practical clinical question. We selected it as a model of what is needed more in today's clinical research landscape.
The authors
The first author of the trial, Vincent Rajkumar, is a Professor of Medicine at Mayo Clinic Rochester. He is a pioneer in the field of multiple myeloma and has been involved in several clinical trials. He is also very active on social media. Check out his profile and website!
Here is the paper, also available here.