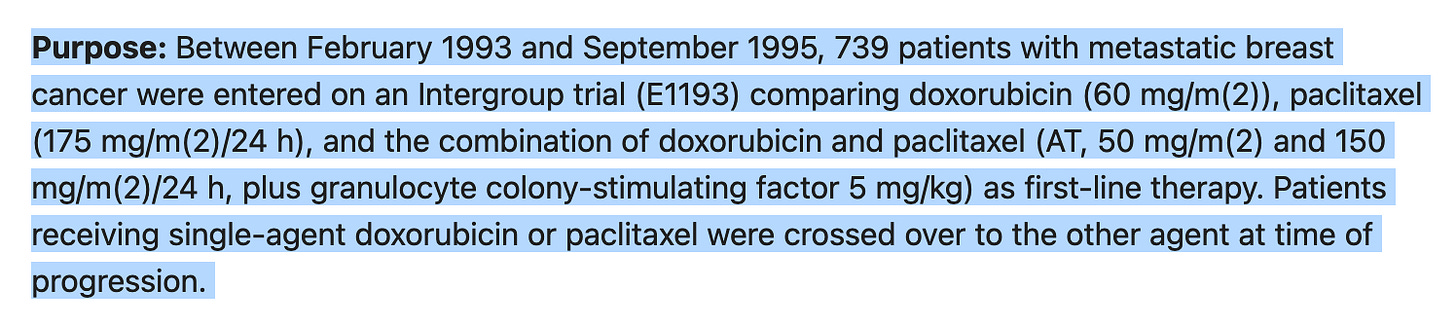
FRIDAY'S GEM: overall survival in first-line metastatic breast cancer.
E1193, an intergroup trial led by George Sledge, with survival results driving the main conclusions of the study.
FRIDAY’S GEM, EDITORS’S PICK
In this section, every week, we present a paper that cought our attention, it can be recent, seminal, historical, a point of view, a commentary, a clinical trial, any publication that we feel is important!
The E1193 trial is an example of a practice-informing trial, which serves as a refreshing reminder of how to interpret or incorporate novel findings.
Strenghts
A very pragmatic trial answering a day-to-day clinical question: what is the best first-line therapy in patients with metastatic breast cancer: monotherapy or combination treatment ?
Main results
Patients responded to the treatments as follows: 36% for doxorubicin, 34% for paclitaxel, and 47% for the combination of doxorubicin and paclitaxel. The combination had a significantly higher response rate.
The median time to treatment failure (TTF) was 5.8 months for doxorubicin, 6 months for paclitaxel, and 8 months for the combination. In other words, the combination had significantly longer TTF than the other two treatments.
The average survival times were 18.9 months for doxorubicin, 22.2 months for paclitaxel, and 22 months for the combination therapy. These differences were not significant. Overall quality of life after 16 weeks of treatment was similar for all three groups.
The Gem
What is really refreshing is that a reasonable conclusion was drawn:
YES: the combination was more active (response rate)
YES: it allowed longer TTF
but NO: survival and quality of life were no better.
In modern days, comparable results—i.e., PFS-only gains—would probably lead many experts, guidelines, and key opinion leaders (KOL) to advocate for a change in practice based on such a trial. In breast cancer, stay tuned for our upcoming analysis of the DESTINY-Breast06 trial, which will be presented at ASCO 2024.
What could explain such a change over time? One graph may explain it. In this paper led by Del Paggio, we can see that the clinical trials landscape in oncology has progressively been dominated by the industry over the last decades. Without saying this is the only reason, this is likely a powerful explanation of why the mentality has changed so profoundly in our field.
The authors
The lead author, George Sledge, is a a Professor of Medicine - Oncology at Stanford. He is a clinician and a pioneer in the field of breast cancer research. He has led or participated to several key trials in the field, including the one we presented today. Check-out this fascinating interview in the Plenary Session podcast.
Here are some quotes from the interview:
"The biggest tragedy is not that our drugs don't work very well, that [itself] is a tragedy, [but] the biggest tragedy is that we don't know who they're going to work in. And so we expose a significant number of patients to untoward toxicity with no benefit whatsoever." - Dr. Sledge
and let’s conclude with this more positive quote :
"I love the idea of thinking through medical problems and thinking them through in terms of how they'd apply to patients" - Dr Sledge