FDA's censoring rules doubled the PFS difference, how is this possible?
When different censoring rules shape different trial results, there is a problem.
Just out in the European Journal of Cancer, we analysed a trial where applying censoring rules favored by the FDA – which censors more patients – doubled the PFS difference.
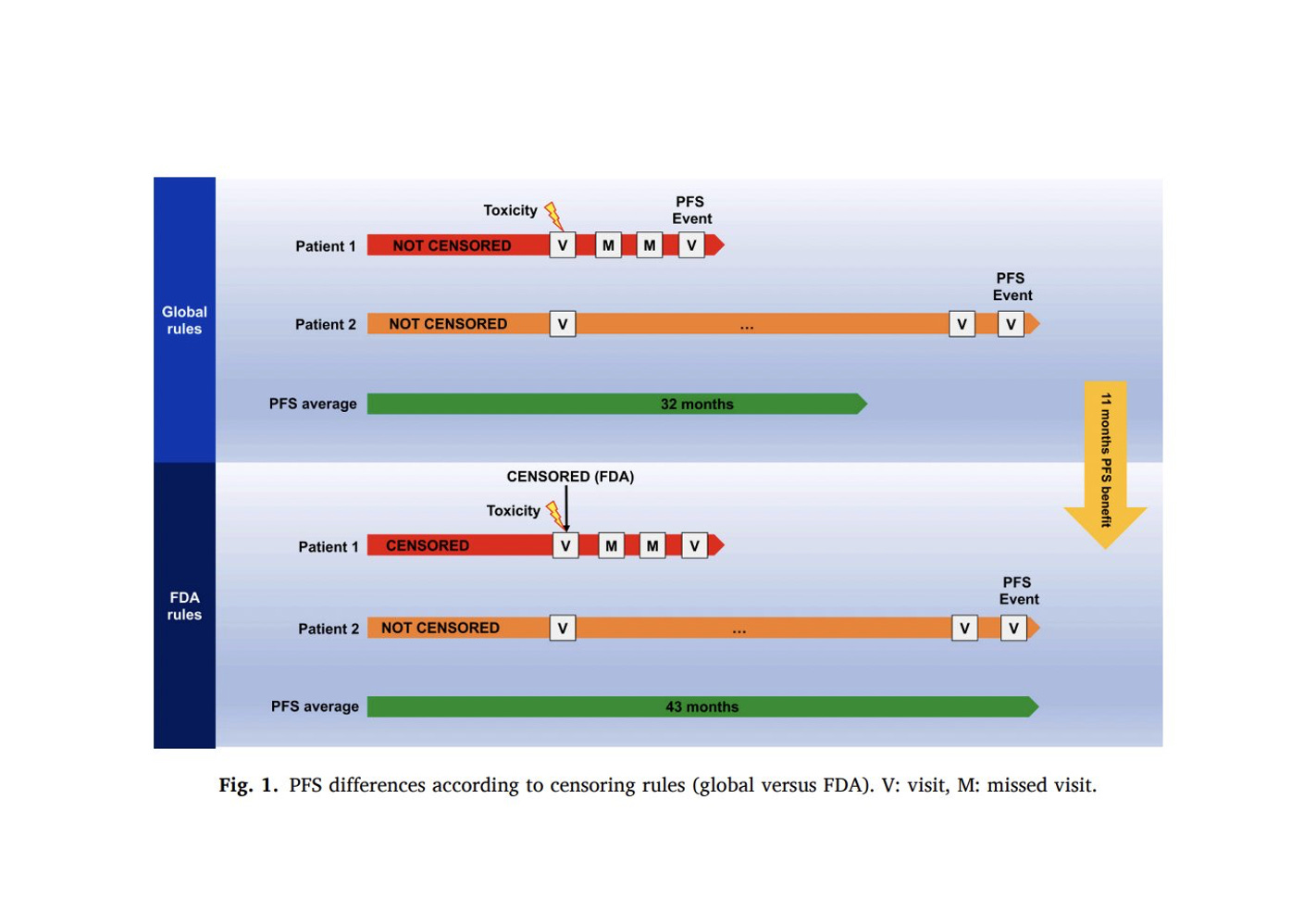
The SYMPATICO trial investigated the combination of venetoclax plus ibrutinib versus placebo plus ibrutinib in patients with relapsed/refractory mantle cell lymphoma. PFS was the primary endpoint. By the FDA censoring rules, the median PFS in the experimental arm increased from 32 to 43 months… the PFS benefit doubled!
How is this possible?
The “only way to improve PFS when you censor more patients is if the censored patients are doing worse than those who remain uncensored”.
Let’s imagine a study with only two patients in the experimental arm.
- Upper panel = global censoring rules = both patients uncensored.
- Lower panel = FDA censoring rules = one patient censored.
The person censored by the FDA (red bar lower panel) must have had early progression. If the FDA does not include this patient in the analysis, the average PFS naturally increases (longer green bar, lower panel). This explains why the PFS benefit doubled (+ 11 months between upper green bar and lower green bar). Interestingly, the control arm remained unaffected by the changing rules, which is a proof, in and of itself, that censoring was informative.
When such phenomenon occur, the results are volatile, fragile, and poorly reliable.
Informative censoring can distort trial results. We draw several lessons and implications for PFS as an endpoint in cancer randomized trials in the paper.
Full access here, co-authored with Vadim Lesan and Timothée Olivier.