FDA "agnostic" approvals in rare cancers: highlighted by trastuzumab deruxtecan – targeting HER2 – in patients with sarcoma
It’s my pleasure to introduce this guest commentary by my UCSF colleague Brian Schulte. It concerns a recent paper he authored with the co-editor of the drug development letter Timothee Olivier. The US FDA recently authorized Traz-Deruxtecan to anyone with a HER2 3+ IHC, regardless of the tissue of origin. Brian discusses the implications of this so-called tumor agnostic approval for sarcoma.
Our goal was not merely highlighting which ‘oma’ was omitted from the latest pan-tumor approval. These observations and subsequent commentary came from a detailed look at the destiny trials filtered through a lens of awareness of the history of HER2 and the unfortunate failures to target HER2 in the myriad subtypes of sarcoma that fall into our tiny domain. The role of the FDA is often unenviable, and sitting back and sniping would not be productive.
It is understandable that an admixture of efficacy data, limited though it may be in some instances, was shaped through good intentions into an accelerated approval of trastuzumab deruxtecan (TDXd) for advanced solid tumors. After all, we don’t see a 51% response rate every day. Nonetheless, especially for wide-spanning approvals, we need to be honest and thorough with the trial data so that we might make informed decisions as oncologists. Increasingly, and perhaps because of the growing complexity of our specialty, many of the results of these studies may be interpreted as ‘your mileage may vary’ (YMMV). Thankfully, much of the patient data underlying the pan-tumor approval of TDXd were available. This lets us see if TDXd has similar efficiency on our sarcoma backroads.
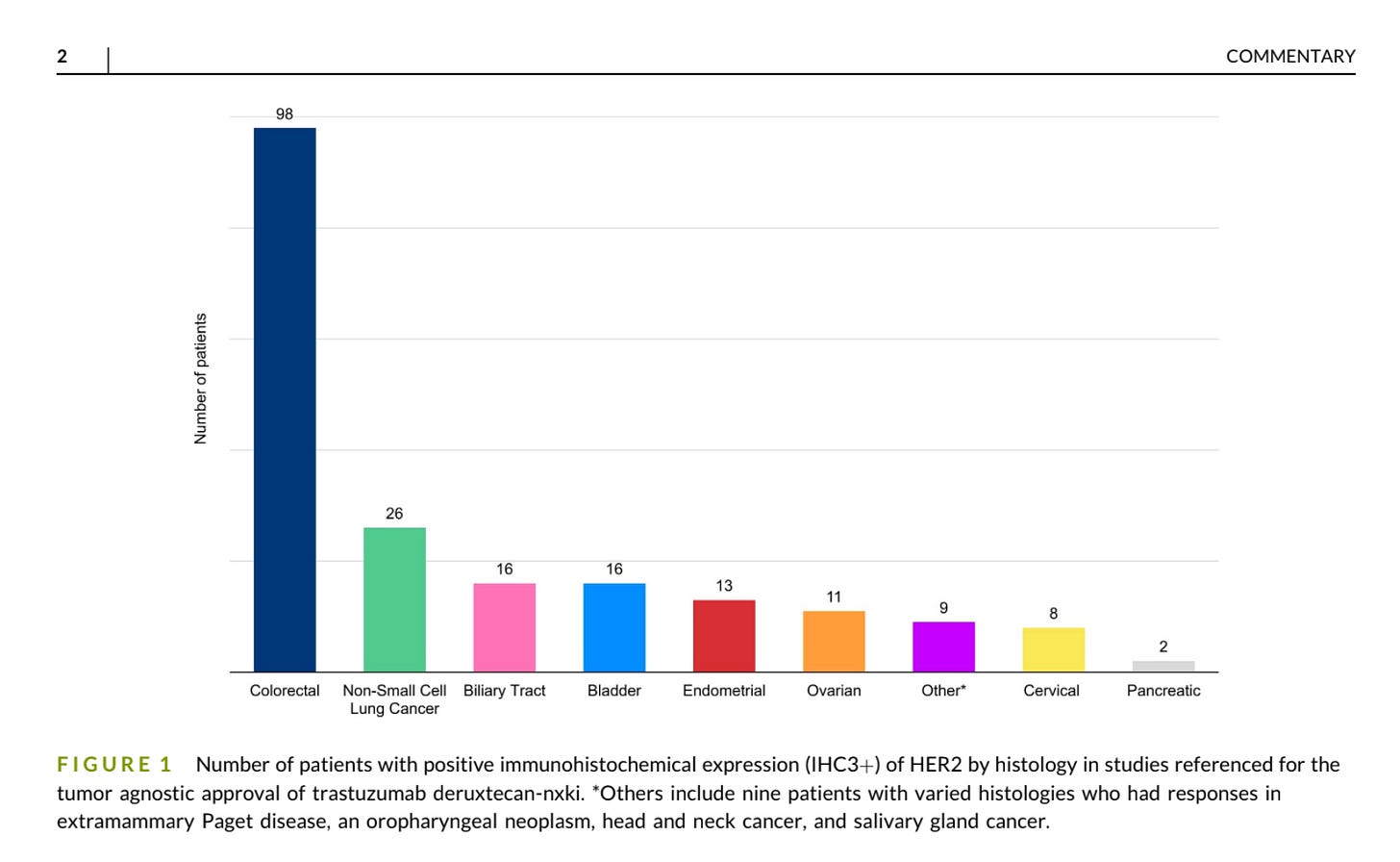
Under the hood, we observed no sarcomas within referenced studies for accelerated approval with positive (IHC3+) staining for HER2. A single patient with an extraskeletal myxoid chondrosarcoma with IHC1+ staining included in the phase 1 study had stable disease as best response. And so, we have, at best, an absence of evidence for TDXd in sarcomas here. Yet, these data stand in the foreground of a small arena littered with the corpses of failed trials of HER2-directed cellular therapies, antibodies, and antibody-drug conjugates (including TDXd itself) for sarcomas.
Moreover, historically, there has not been a standard means of measurement of HER2 IHC in sarcomas. Prior studies have evaluated both membranous and cytoplasmic expression. Where HER2 resides in the cell may have differential implications for TDXd efficacy. The sole responding patient in PEPN1924 had only 15% expression. There is probably something we’re missing here. This is bolstered by broader questions about the interpretation of and role of HER2 status as it pertains to assays like Herceptest, the standard measure for HER2.
We recommend a degree of caution around TDXd for patients with sarcomas. Broad and early administration of TDXd may incur a true opportunity cost if administered to patients who might otherwise qualify for studies.
That said, TDXd is an active agent in a large swath of solid tumors. Whether sarcomas are included among them remains to be seen.





There's a lot left unsaid here. Should the lack of efficacy data in HER2+ have been used to curtail the pan-tumor accelerated approval? Is it really robust enough to know that HER2 directed therapy doesn't work in Sarcoma? Does limiting the available armamentarium help patients? Are these tradeoffs the FDA is compelled to evaluate?