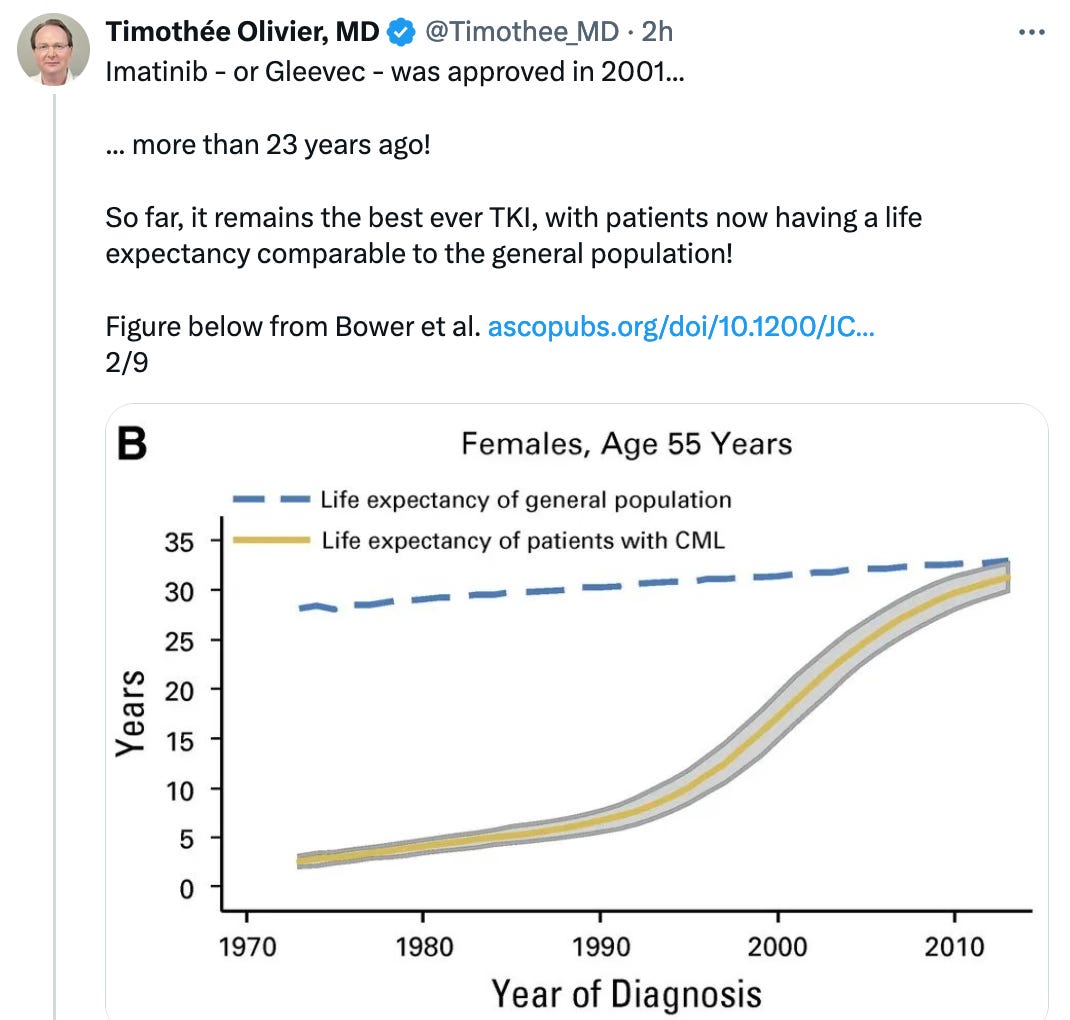
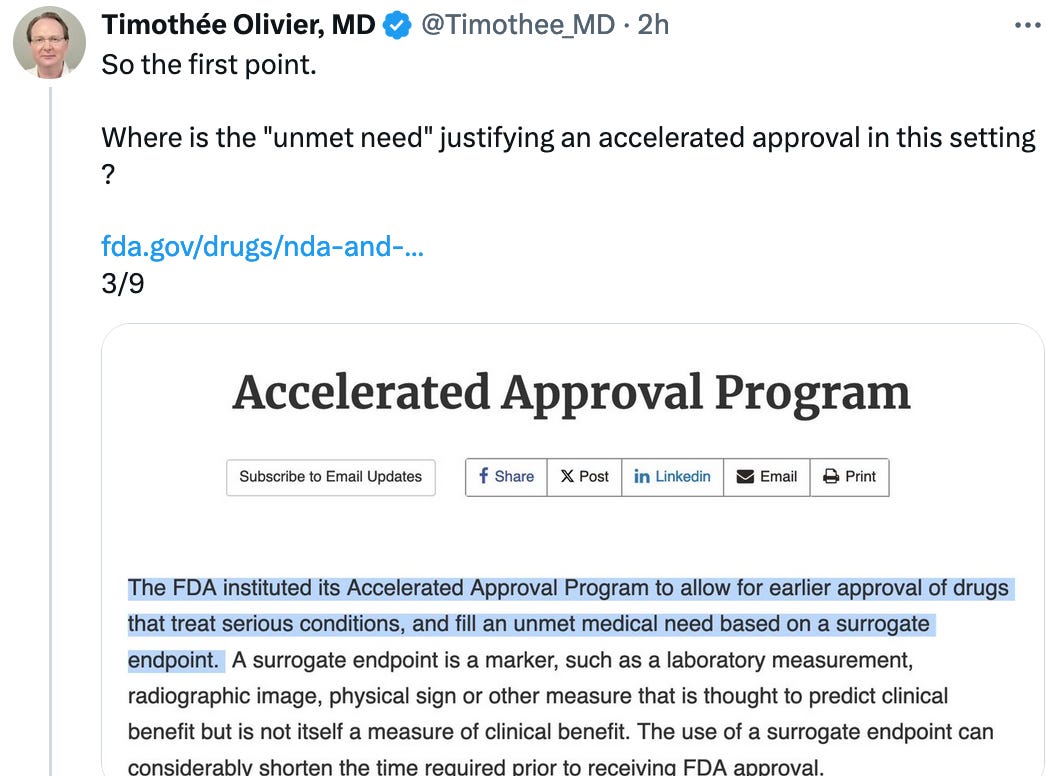
After 23 years, imatinib remains the best front-line therapy in CML. Why use accelerated approval in a setting where patients have a life expectancy comparable to the general population?
Asciminib - a novel TKI - in Newly Diagnosed Chronic Myeloid Leukemia.
Here is a thread I posted on X after asciminib was approved by the US FDA under the accelerated pathway.
References:
Below are the links to:
the X thread
our recent publication led by Nethra Srinivasan
the FDA accelerated approval of asciminib
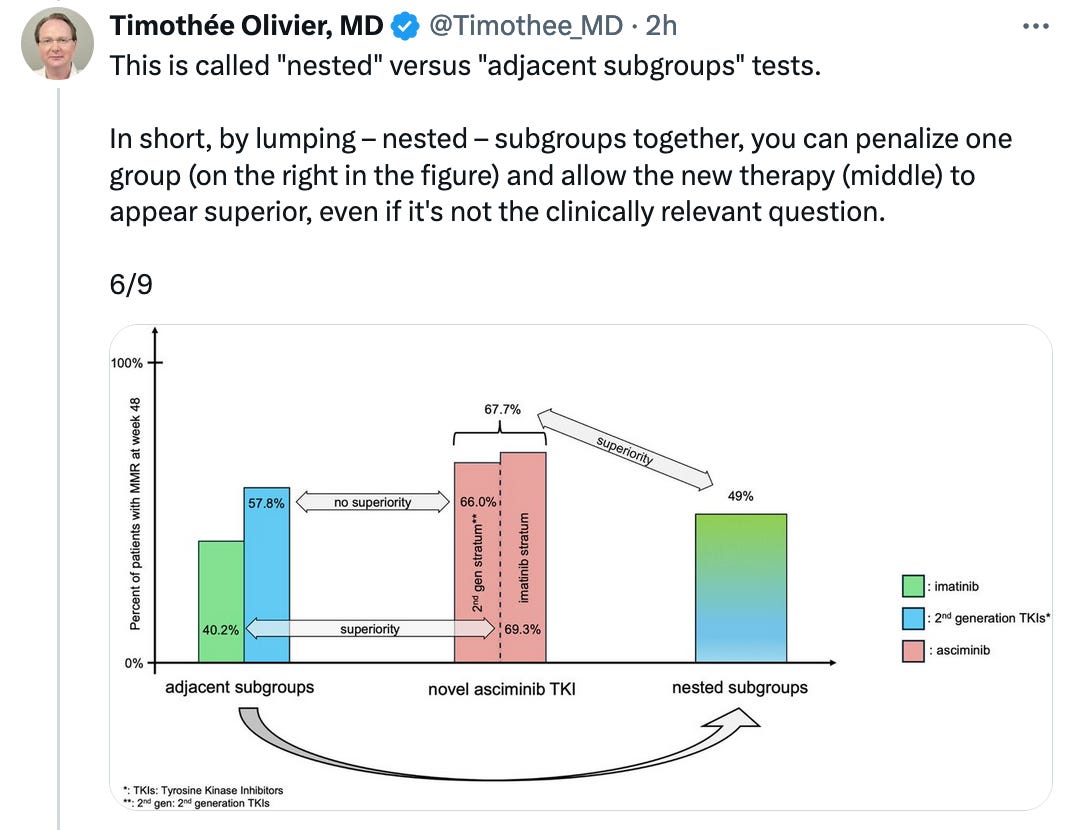
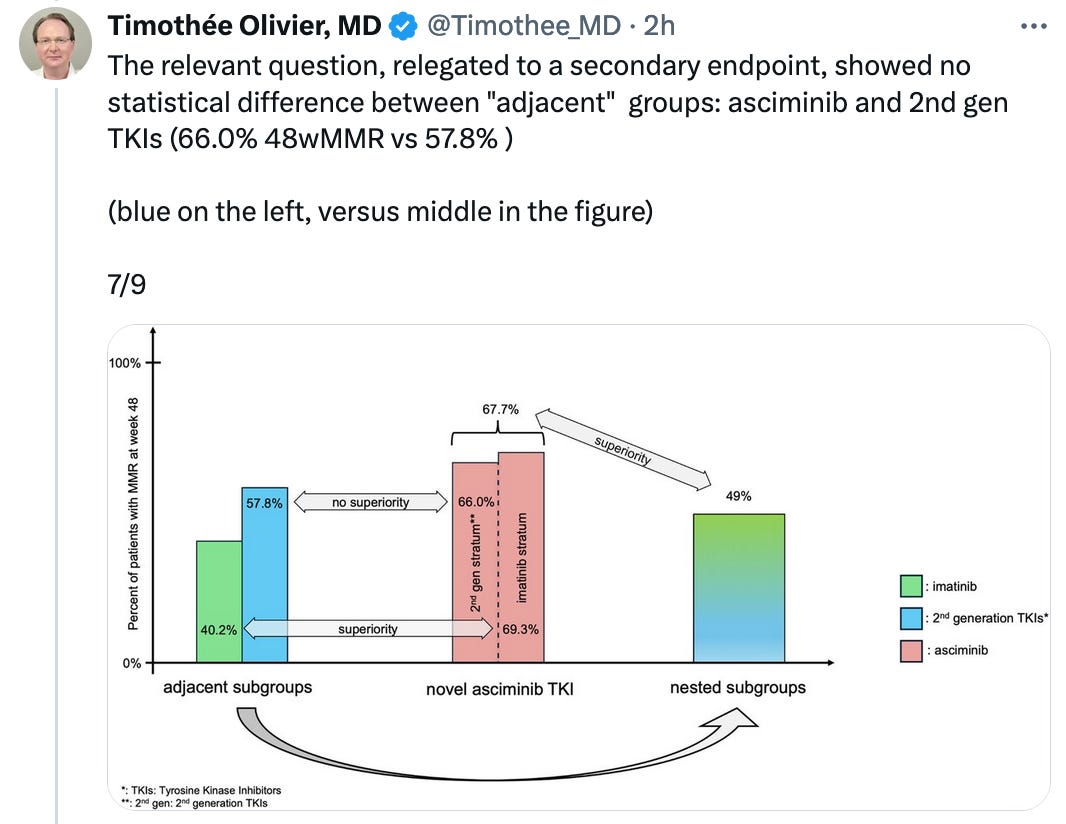
nested and adjacent subgroups publication led by Sunny Kim
two papers by Anushka Walia and Vinay Prasad about limitations of molecular milestones and related topics: here and here.







Great post. Thank you